Research Funded Pilot Projects
5% Random Sample of the Medicare Enrollment and Claims Data
Daniela Moga, MD, PhD
Associate Professor, Pharmacy Practice & Science
FY23 COP Equipment Pilot award, October 2022
Medicare is the federal health insurance program for US adults meeting one of the following criteria: (1) age (≥65 years), (2) select disabilities regardless of age, and (3) end-stage renal disease regardless of age. The majority of the Medicare enrollees qualify based on the age criterion and nearly 98% of the US older adult population (≥65 years) has Medicare insurance. The Centers for Medicare and Medicaid Services grants researchers paid access to Medicare administrative claims databases for research purposes. Available data for research include enrollment information along with beneficiaries’ encounters with the health care system and receipt of therapeutic interventions (i.e., medications, procedures, and services). CoP has acquired a 5% random sample of the Medicare enrollment and claims data (2012-2021) for Medicare enrollees ≥65 years to evaluate medication use and the effects of potentially inappropriate medications use in this population. As access is restricted by a data use agreement, researchers interested in the use of these data should contact Dr. Daniela Moga for additional information.

Waters Arc HPLC with PDA and fluorescence detector
Steven Van Lanen, PhD
Professor, Pharmaceutical Sciences (College of Pharmacy)
FY23 COP Equipment Pilot award, October 2022
The Waters Arc High Performance Liquid Chromatography (HPLC) system is designed for high-efficiency separations of analytes with low carryover, high injection precision, and high back pressure tolerance. The system configuration includes quaternary solvent delivery with temperature control. The HPLC is controlled using Empower Chromatography Data System and is equipped with photodiode array and fluorescence detectors for analyte detection.

College of Pharmacy Equipment Pilot: Malvern Zetasizer Pro
Vincent Venditto, PhD
Assistant Professor, Pharmaceutical Sciences (College of Pharmacy)
FY22 COP Equipment Pilot award , April 2022
The Zetasizer Pro is the industry standard instrument for the measurement of protein and nanoparticle size, molecular size, electrophoretic mobility, zeta potential, and molecular weight. The instrument uses dynamic light scattering and electrophoretic light scattering enables characterization across a breadth of applications. The user-friendly interface enables rapid sample characterization for routine measurements by personnel with training at the high school level and up.

Urinary immune cell profiling in sepsis-associated acute kidney injury
Alexander Flannery, PharmD, PhD, FCCM, BCCCP, BCPS
Assistant Professor, Pharmacy Practice and Science (College of Pharmacy)
Clinical Research Catalysts (CRC) Pilot Award, July 2021 - present
Up to 50% of cases of acute kidney injury (AKI) are attributed to sepsis, which is a life-threatening organ dysfunction caused by a dysregulated host response to infection. Combining their expertise in sepsis-AKI research and immunology, Drs. Flannery and Feola are collaborating to characterize the urinary immune cell profile as it evolves over time in patients with sepsis-associated AKI to better understand the potential role of various immune cells in kidney injury and repair, particularly macrophages. Additionally, the pilot will allow them to refine techniques to study gene expression at the single cell level with scRNA-seq technology. Together, these studies will use non-invasive urine samples to forge an improved understanding of the immunobiology in various stages of kidney injury and repair due to sepsis.
Co-PI: David Feola, PharmD, PhD (Pharmacy Practice and Science)
Mechanistic and pharmacologic studies of selective mithramycin analogues targeting EWS-FLI1 in Ewing sarcoma
Markos Leggas, PhD
Professor, Pharmaceutical Science (College of Pharmacy)
Markey Run for the Roses Award, January 2021 - present
Ewing sarcoma family of tumors (ESFT) is a family of resilient devastating cancers of bone and soft tissue affecting primarily children and young adults. Current highly cytotoxic combination therapy of five drugs provides only 30% overall survival. The goal of this project is to gain molecular insights into the mode of action of MTM via structural, biochemical and pharmacological studies to generate a highly efficacious and selective anti-ESFT treatment. The Markey Run for the Roses Award is a one-time 10% match of the YR1 direct costs (up to $40K) for a new multi-PI NCI R01 grant.
Co-PIs: Jurgen Rohr, PhD (Pharmaceutical Sciences); Oleg Tsodikov, PhD (Pharmaceutical Sciences)

Anti-inflammatory and gingival absorption properties of a new mPGES-1 inhibitor
Octavio Gonzalez, DDS, MS, PhD
Associate Professor, College of Dentistry
Igniting Research Collaborations (IRC) Pilot, January 2021 - present
Periodontal disease is a chronic inflammatory disease that, if not treated, leads to local (i.e., tooth loss) and systemic effects (increased risk for cardiovascular disease, poor diabetes control, and Alzheimer’s disease). Microsomal prostaglandin E synthase-1 (mPGES-1) is known as a promising target for development of a next generation of anti-inflammatory drugs. Through structure-based de novo drug design, the Zhan Lab has designed and discovered a novel type of potent and highly selective mPGES-1 inhibitors targeting the conserved region of the active site that are suitable for preclinical studies. In this pilot, the Gonzalez lab will evaluate the potential of a novel, potent and highly selective mPGES-1 inhibitor in treatment of periodontitis and the Zhan lab will conduct inflammatory biomarker analysis.
Co-PIs: Chang-Guo Zhan, PhD (Pharmaceutical Sciences)
Opioid prescribing in dentistry: Building and implementing a framework to nudge provider behavior
Douglas Oyler, PharmD
Assistant Professor, Pharmacy Practice & Science (College of Pharmacy)
Igniting Research Collaborations (IRC) Pilot, January 2021 - present
This project aims to evaluate patient- and provider-specific factors related to opioid prescriptions after dental extractions through medical record analysis and qualitative interviews with prescribers. Additionally, the project will use state-level prescription drug monitoring program data to build a framework for evaluation of future electronic medical record interventions to address opioid prescribing in dentistry. This collaboration leverages expertise from Dentistry, Medical Informatics, Public Health, Behavioral Science, and Opioid Safety to address an ongoing public health concern in Kentucky.
Co-PIs: Marcia Rojas-Ramirez, PhD (College of Dentistry), Hilary Surratt, PhD (Behavior Science, College of Medicine), Dana Quesinberry, JD, DrPH (KIPRC, College of Public Health), Philip Bernard, MD (Pediatrics, College of Medicine), Craig Miller, DDS, MS (Oral Diagnosis, College of Dentistry)

Cognitive reserve and driving mobility
Caitlin Pope, PhD
Assistant Professor, Department of Health, Behavior & Society (College of Public Health)
Igniting Research Collaborations (IRC) Pilot, January 2021 - present
In the United States, Alzheimer’s disease and related dementias (ADRD) are the 5th leading cause of death for adults 65 and older and are estimated to affect approximately 13.8 million adults in 2050. One proposed mechanism of maintaining cognitive abilities in the face of brain neuropathology over time is cognitive reserve. To date, there is a lack of research on cognitive reserve and functional abilities such as driving mobility and if any differences seen in driving mobility in early stages of cognitive impairment can be attributed to cognitive reserve. This project will examine the association between cognitive reserve and driving mobility in older adult drivers across a continuum of cognitive impairment.
Co-PIs: Daniela Moga, MD, PhD (Pharmacy Practice & Science); Yang Jiang, PhD (Behavioral Neuroscience, College of Medicine)

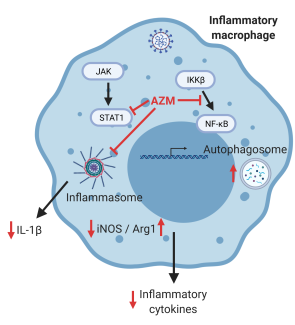
A study of macrophage gene expression in sarcoidosis and the effect of azithromycin derivatives
Parijat Sen, MD
Assistant Professor, Internal Medicine (College of Medicine)
Igniting Research Collaborations (IRC) and COBRE for Translational Chemical Biology co-Pilot, January 2021 - present
Sarcoidosis is a multi systemic granulomatous inflammatory condition affecting more than 200,000 Americans and many more globally. Due to a lack of clear understanding of the involved immune pathways, current therapies are mostly limited to various systemic immunosuppressive agents. This project aims to explore the effect of azithromycin, an FDA-approved antibiotic with immunomodulatory properties, on gene expression in macrophages (a predominant inflammatory cell forming sarcoid granulomas) through transcriptomic assays. The pilot also aims to explore a library of synthetic derivatives of azithromycin to try and identify compounds which retain the immunomodulatory effect on macrophages but lack antimicrobial properties. This project involves a diverse team with expertise in clinical care of sarcoidosis, immunology and biosynthetic molecular development to identify novel targets and molecules for sarcoid therapy.
Co-PIs: David Feola, PhD, PharmD (Pharmacy Practice & Science); Jamie Sturgill, PhD (Internal Medicine, College of Medicine), Steven Van Lanen, PhD (Pharmaceutical Sciences)

Early reduction of Post-Operative Pain and inflammation to Expedite Return to function after KNEE arthroscopy (PROPER KNEE trial)
Austin Stone, MD PhD
Assistant Professor, Orthopaedic Surgery (College of Medicine)
Igniting Research Collaborations (IRC) Pilot, January 2021 - present
Meniscus tears requiring surgery occur in 15% of Americans ages 10-65 years, which makes arthroscopic meniscal procedures the most commonly performed orthopaedic surgery in both civilian and military populations. It is common to use an opioid drug for post-operative pain relief. Through development and use of a unique drug repurposing approach, called DREAM-in-CDM (Drug Repurposing Effort Applying Integrated Modeling-in vitro/vivo-Clinical Data Mining), the Zhan Lab has identified a potent mPGES-1 inhibitor (targeting the open conformation of mPGES-1 predicted computationally) among existing FDA-approved non-opioid drugs. In this project, a pilot clinical trial will be conducted by a very talent multidisciplinary team to test the efficacy of the non-opioid drug in treatment of post-operative pain.
Co-PIs: Chang-Guo Zhan, PhD (Pharmaceutical Sciences); Lauren Whitehurst, PhD (Psychology, College of Arts & Sciences), Cale Jacobs, PhD (Orthopaedic Surgery, College of Medicine), Nicole Cascia, PhD, CES (Orthopaedic Surgery, College of Medicine)
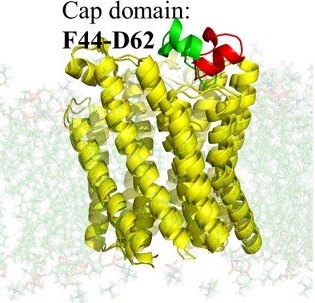
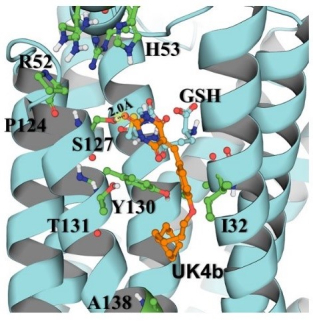
Novel mith-platin conjugates for treatment of platinum-complex resistant ovarian cancers
Jill Kolesar, PharmD, MS, FCCP, BCPS
Professor, Department of Pharmacy Practice & Science
Inter-Departmental Collaboration (IDC) Pilot, July 2019 - present
More than 20,000 women will be diagnosed with, and approximately 14,000 will die of, ovarian cancer in 2021. To address this unmet medical need, Drs Kolesar, Rohr and Tsodikov are working together to develop new medications for treating ovarian cancer, mithplatins. Mithplatins overcome common resistance mechanisms in ovarian cancer and will be synthesized, tested for DNA binding and damaging capabilities and evaluated in preclinical models. This highly innovative work introduces an entirely novel class of compounds that are both clinically relevant and mechanistically important, providing key insights into the action of mithplatins on DNA binding and repair.
Co-PIs: Jurgen Rohr, PhD (Pharmaceutical Sciences); Oleg Tsodikov, PhD (Pharmaceutical Sciences

Contact Us
The Office of Research Operations provides programs, support services, and infrastructure to enable the pioneering research of our faculty, the diverse portfolio of which spans basic, translational, health outcomes, and public policy research. Contact us to learn more.